Describe the Characteristics of a Polar Molecule
1 Polar molecules should have opposite charge. The oxygen side of the molecule features.
Polar Molecule Definition And Examples Biology Dictionary
- they should have same dipole moment value.
. Density - the amount of thickness in a substance. A hydrogen bond a special attraction between some polar molecules is another important characteristic. See examples of polar.
This is due to the shape of the molecule. Drag each of the following characteristics listed below to the molecule they describe either DNA or RNA. Polar molecules tend to dissolve well in water and other polar solvents.
Select the correct answer below. A polar molecule is a molecule that has a mostly positive charge on one side and a mostly negative charge on the other. - they should have opposite charge.
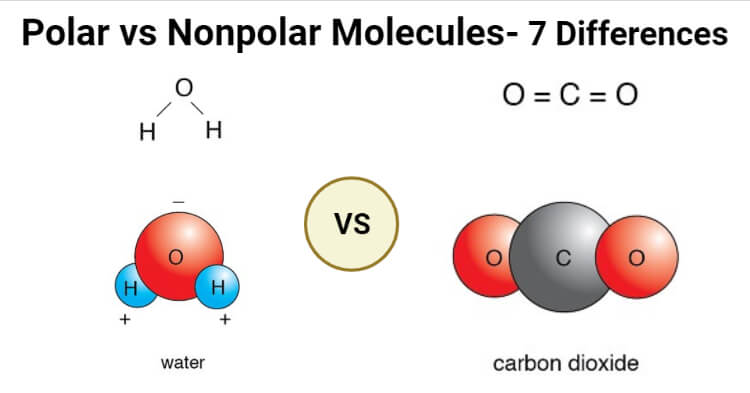
The non polar molecule is electrically neutral and stable. Also what are the characteristics of molecules. Water is a polar molecule and also acts as a polar solvent.
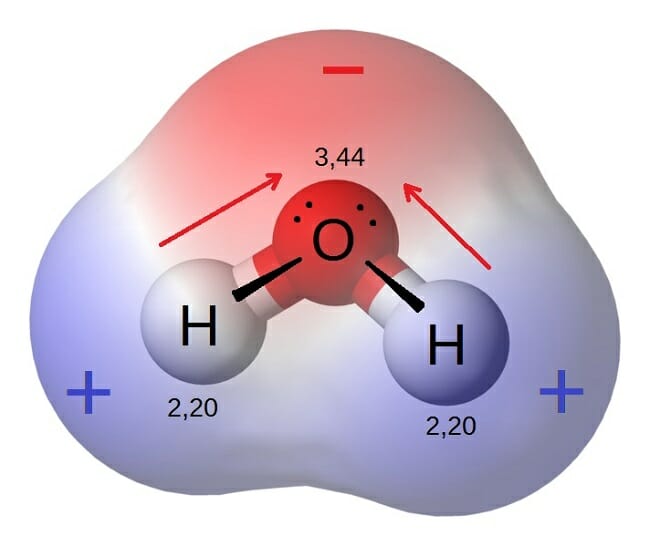
Polar Molecule Definition. Examples of Molecules with Polar Covalent Bonds. The electronegativity value for oxygen is 344 whereas the electronegativity value for hydrogen is 220.
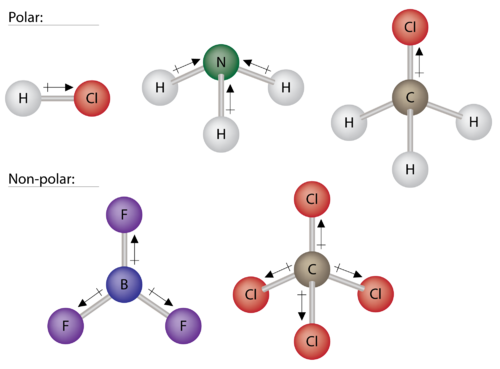
Have a polar head and a nonpolar tail Steroids. When a chemical species is said to be polar this means that the positive and negative electrical charges are unevenly distributed. Polar and non-polar molecules.
The side of the molecule with the lone pair is slightly negative while the side with the 3 Hs is slightly positive due to the differences in electronegativity described above. The difference in the distribution of electrons accounts for the best shape of the molecule. Its the movement of electrons that determines polarity.
Explain the conditions under which this molecule could cross by simple diffusion. They must be uncharged non polar substance and also consist of hydrophobic nature. Describe the characteristics of a molecule that could cross a cell membrane 1.
There are also amphiphilic molecules large molecules that have both polar and nonpolar groups attached to them. Describe the characteristics of lonic electrolytes Question Which of the following best describes the term dissociation. For example water is.
O Dissociation is the electrostatic attraction between an ion and a polar molecule. Polarity is a description of how different the electrical poles of a molecule are. Polar molecules usually have a higher boiling and melting point as well as a high surface tension as polar linkages are considerably stronger than nonpolar linkages.
A polar molecule is a type of molecule that has a separation of electric charge where one side of the molecule is positively charge and the other side is negatively charged. Molecules can also be non polar. The positive charge comes from the atomic nucleus while the electrons supply the negative charge.
A polar molecule is a chemical species in which the distribution of electrons between the covalently bonded atoms is not even. Includes the sex hormones are hydrophobic have a back bone of four fused carbon rings. Polar molecule is characterized by an uneven distribution of the electrons that form the covalent bonds between each atoms in the molecule.
Water or H2O is an example of a polar. This occurs because of the differences in electronegativity between atoms of different elements. Electronegativity of O 35 The resulting molecule is considered polar where oxygen becomes slightly negative and the hydrogen becomes slightly positive.
The molecule as a whole is also polar because the things around it though arranged in a tetrahedral pattern are not all the same. - they should have electronegativity different. Difference in electronegativity 21 - 19 02 From above some difference in the electronegativities of the atoms is there.
These all characteristics help them in moving down the concentration gradient. A polar molecule is composed of a positive charge and a negative charge. Some examples of polar molecules are H2O CHF3 NH3 etc.
Some characteristics of polar molecules are discussed below. Examples include O 2 N 2 and F 2 You can use the following chart to predict the type of bond. Classify the following characteristics listed on the right by dragging each to the type of lipid.
This charge difference allows the positive end of the molecule to attract to the negative end of another. What are the characteristics of a polar covalent molecule. If they are highly different it can be said that the species is a highly polar molecule.
Water H2O is a molecule having a polar covalent bond. Polar covalent bonds are a type of covalent bond and means unequal sharing of electrons. Explore the polar molecule in chemistry.
Polar molecule molecule with an unequal distribution of charge resulting in the molecule having a positive end and a negative end element any substance that cannot be broken down into simpler substances atom The smallest component of an element having the chemical properties of the element nucleus the center of an atom isotopes. 1 on a question Water is referred to as a polar molecule. Characteristics of polar molecule.
In polyatomic compounds polarity depends on the net dipole moment of all the covalent bonds present in the molecule. 3 They should have electronegativity difference. 2 They should have some dipole moment value.
The correct answer is polar tetrahedral and non-polar. A substance that contains polar covalent bonds may not be overall polar. Some chemical species such as chains.
Learn about its characteristics and how to determine the polarity of a molecule. O Dissociation is the extent to which a covalent solute may be dissolved in a solvent. A polar molecule is characterized by the uneven distribution of the electrons that form the covalent bonds between each atom in the molecule resulting in a slightly positively charged side and a slightly negatively charged side.
Characteristics of polar molecules. In a polar molecule one side of the molecule has a positive electrical charge and the other side has a negative electrical charge. It is given a compound which is silicon tetrahydride having formula The electronegativities of silicon and hydrogen are 19 and 21 respectively.
Describe the characteristics of a polar molecule.
Polar Vs Nonpolar Molecules Definition 7 Key Differences Examples

Polar Molecule Characteristics Example What Makes A Molecule Polar Video Lesson Transcript Study Com
Comments
Post a Comment